Call & WhatsApp: +91-73375-30225
24/7 Email: bonhoahealth@gmail.com
No products
Prices are tax excluded
Indications & Dosage
Veenat (Imatinib) is a kinase inhibitor indicated for the treatment of:
- Newly diagnosed adult and pediatric patients with Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in the chronic phase
- Patients with Ph+ CML in blast crisis (BC), accelerated phase (AP), or in the chronic phase (CP) after failure of interferon-alpha therapy
- Adult patients with relapsed or refractory Ph+ acute lymphoblastic leukemia (Ph+ ALL)
- Pediatric patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) in combination with chemotherapy
- Adult patients with myelodysplastic/myeloproliferative diseases (MDS/MPD) associated with PDGFR (platelet-derived growth factor receptor) gene rearrangements as determined with an FDA-approved test
- Adult patients with aggressive systemic mastocytosis (ASM) without the D816V c-KIT mutation as determined with an FDA-approved test or with c-KIT mutational status unknown
- Adult patients with hypereosinophilic syndrome (HES) and/or chronic eosinophilic leukemia (CEL) who have the FIP1L1-PDGFRα fusion kinase and for patients with HES and/or CEL who are FIP1L1-PDGFRα fusion kinase negative or unknown
- Adult patients with unresectable, recurrent, and/or metastatic dermatofibrosarcoma protuberans (DFSP)
- Patients with KIT (CD117)-positive gastrointestinal stromal tumors (GIST) that cannot be surgically removed and/or have spread to other parts of the body
- Adult patients after surgery who have had their KIT (CD117)-positive GIST completely removed
DOSAGE AND ADMINISTRATION
- Adults with Ph+ CML CP: 400 mg/day
- Adults with Ph+ CML AP or BC: 600 mg/day
- Pediatrics with Ph+ CML CP: 340 mg/m2 /day
- Adults with Ph+ ALL: 600 mg/day
- Pediatrics with Ph+ ALL: 340 mg/m2 /day
- Adults with MDS/MPD : 400 mg/day
- Adults with ASM: 100 mg/day or 400 mg/day
- Adults with HES/CEL: 100 mg/day or 400 mg/day
- Adults with DFSP: 800 mg/day
- Adults with metastatic and/or unresectable GIST: 400 mg/day
- Adjuvant treatment of adults with GIST: 400 mg/day
- Patients with mild to moderate hepatic impairment: 400 mg/day
- Patients with severe hepatic impairment: 300 mg/day
All doses of Gleevec should be taken with a meal and a large glass of water. Doses of 400 mg or 600 mg should be administered once daily, whereas a dose of 800 mg should be administered as 400 mg twice a day. Imatinib can be dissolved in water or apple juice for patients having difficulty swallowing. Daily dosing of 800 mg and above should be accomplished using the 400 mg tablet to reduce exposure to iron.
Imatinib Side effect:
- Edema and severe fluid retention have occurred. Weigh patients regularly and manage unexpected rapid weight gain by drug interruption and diuretics.
- Cytopenias, particularly anemia, neutropenia, and thrombocytopenia, have occurred. Manage with dose reduction, dose interruption, or discontinuation of treatment. Perform complete blood counts weekly for the first month, biweekly for the second month, and periodically thereafter.
- Severe congestive heart failure and left ventricular dysfunction have been reported, particularly in patients with comorbidities and risk factors. Monitor and treat patients with cardiac disease or risk factors for cardiac failure.
- Severe hepatotoxicity including fatalities may occur. Assess liver function before initiation of treatment and monthly thereafter or as clinically indicated. Monitor liver function when combined with chemotherapy known to be associated with liver dysfunction.
- Grade 3/4 hemorrhage has been reported in clinical studies in patients with newly diagnosed CML and with GIST. GI tumor sites may be the source of GI bleeds in GIST.
- Gastrointestinal perforations, some fatal, have been reported.
- Cardiogenic shock/left ventricular dysfunction has been associated with the initiation of Imatinib in patients with conditions associated with high eosinophil levels (e.g., HES, MDS/MPD and ASM).
- Bullous dermatologic reactions (e.g., erythema multiforme and StevensJohnson syndrome) have been reported with the use of Imatinib.
- Hypothyroidism has been reported in thyroidectomy patients undergoing levothyroxine replacement. Closely monitor TSH levels in such patients.
- Fetal harm can occur when administered to a pregnant woman. Apprise women of the potential harm to the fetus, and to avoid pregnancy when taking Imatinib.
- Growth retardation occurring in children and pre-adolescents receiving Imatinib has been reported. Close monitoring of growth in children under Imatinib treatment is recommended.
- Tumor lysis syndrome. Close monitoring is recommended.
- Reports of motor vehicle accidents have been received in patients receiving Imatinib. Caution patients about driving a car or operating machinery.
- Renal toxicity. A decline in renal function may occur in patients receiving Imatinib. Evaluate renal function at baseline and during therapy, with attention to risk factors for renal dysfunction.
Imatinib Cost in India
Imatinib cost in India or imatinib 100 mg price in India is only 5% of the cost from other countries.
For more Prescribing information,please check the → “FDA Prescribing Information”.
Additional Info
| Package Quantity | 120 |
| Warnings | Don't take the drug without consulting a qualified doctor or physician. |
| Suggested use | Follow the doctor's advice |
| Delivery & Returns | 7-14 days |
Reviews
Glad to be alive
I have been on Generic Imatinib(Gleevec) for a year now. I started with 400mg but could not tolerate it so dropped to 100mg. I am in remission but nausea has become a problem. Taking antinausea meds but still nauseated most of the day. Lorazepam helps but wish I felt better without all of this. Glad to be alive though
Nice online Pharmacy
Praise God for Indian Generic Gleevec/Imatinib.With the exception of diarrhea, bloating and fluid retention, all is great. Am into my second month on the generic Imatinib so we'll see. Again, just so thankful for the online pharmacy's Meds which control the CML and the doctors who developed it.
Trustable online pharmacy
Novartis Gleevec has been effective but since my Medicare coverage changed this year the out of pocket expense of $2k / month makes it not affordable. I must change to Indian generic Imatinib capsules. It seems works and the same as brand one, but price is very very cheap. A trustable online pharmacy.
- 1 out of 1 people found this review useful.
A trial order
See your online pharmacy in Twitter and have bought 1 bottle for a trial order. Hope it is effective. thanks
- 1 out of 1 people found this review useful.
Chronic Myelogenous Leukemia to get new hope
The drug has been effective but since Medicare coverage changed this year the out of pocket expense of $2k / month makes it not affordable. Thanks BonHoa pharmacy to give me hope. Thanks a lot!
- 1 out of 1 people found this review useful.


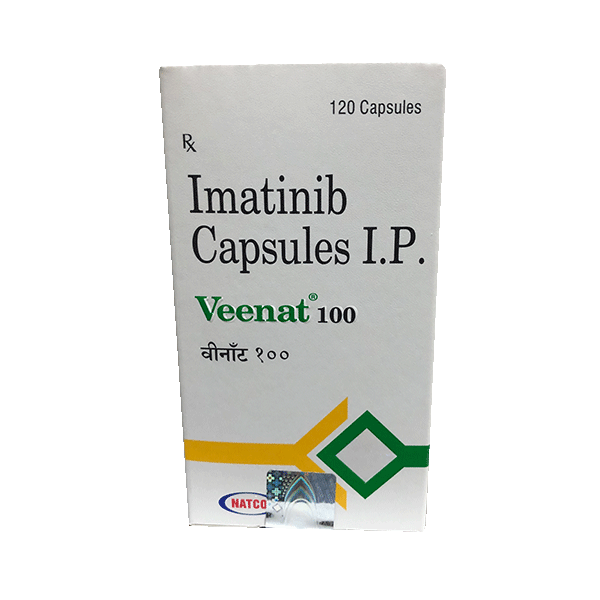





Imatinib price in india
the generic GLEEVEC from BonHoa has extended my survival and I had the honor of meeting Dr Druker who developed the drug, at Oregon Health and Science University. He was absolutely wonderful as was the care I received there. Thanks a lot