Call & WhatsApp: +91-73375-30225
24/7 Email: bonhoahealth@gmail.com
No products
Prices are tax excluded
Indications & Dosage
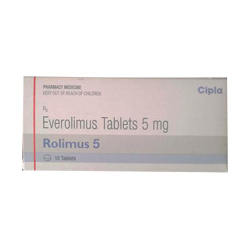
AFINITOR (Everolimus), is a kinase inhibitor indicated for the treatment of:
1.Postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer (advanced HR+ BC) in combination with exemestane after failure of treatment with letrozole or anastrozole.
2.Adults with progressive neuroendocrine tumors of pancreatic origin (PNET) and adults with progressive, well-differentiated, non-functional neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin that are unresectable, locally advanced or metastatic. AFINITOR is not indicated for the treatment of patients with functional carcinoid tumors.
3.Adults with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib.
4.Adults with renal angiomyolipoma and tuberous sclerosis complex (TSC), not requiring immediate surgery.
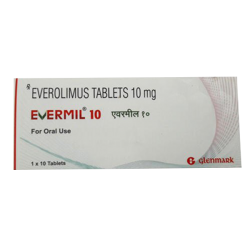
Everolimus are kinase inhibitors indicated for the treatment of:
5.Pediatric and adult patients with tuberous sclerosis complex (TSC) who
have subependymal giant cell astrocytoma (SEGA) that requires therapeutic intervention but cannot be curatively resected.
DOSAGE AND ADMINISTRATION
Advanced HR+ BC, advanced NET, advanced RCC, or renal angiomyolipoma with TSC:
1.10mg once daily with or without food.
2.For patients with hepatic impairment, reducethe AFINITOR dose.
3.If moderate inhibitors of CYP3A4/P-glycoprotein (PgP) are required, reduce the AFINITOR dose to 2.5 mg once daily; if tolerated, consider increasing to 5 mg once daily.
4.If strong inducers of CYP3A4 are required, consider doubling the daily dose of AFINITOR using increments of 5 mg or less.
SEGA with TSC:
1.4.5 mg/m2 once daily; adjust dose to attain trough concentrations of 5-15 ng/mL.
2.Assess trough concentrations approximately 2 weeks after initiation of treatment, a change in dose, a change in co-administration of CYP3A4/PgP inducers or inhibitors, a change in hepatic function, or a change in dosage form between AFINITOR Tablets and AFINITOR DISPERZ.
3.For patients with severe hepatic impairment reduce the starting dose of AFINITOR Tablets or AFINITOR DISPERZ.
4.If concomitant use of moderate inhibitors of CYP3A4/PgP is required, reduce the dose of AFINITOR Tablets or AFINITOR DISPERZ by 50%.
5.If concomitant use of strong inducers of CYP3A4/PgP is required, double the dose of AFINITOR Tablets or AFINITOR DISPERZ.
For more Prescribing information,please check the → “FDA Prescribing Information”.
Additional Info
| Package Quantity | 30 |
| Warnings | Don't take the drug without consulting a qualified doctor or physician. |
| Suggested use | Follow the doctor's advice |
| Delivery & Returns | 7-14 days |







give us new hope
My husband has been on it for 13 weeks with impressive results after failing on sutent and nearly dying from an allergic reaction to torisel. So far, so very good. He has gotten her life back and even traveled overseas for about three weeks. Thanks bonhoa pharmacy give our family new hope.