Call & WhatsApp: +91-73375-30225
24/7 Email: bonhoahealth@gmail.com
No products
Prices are tax excluded
 View larger
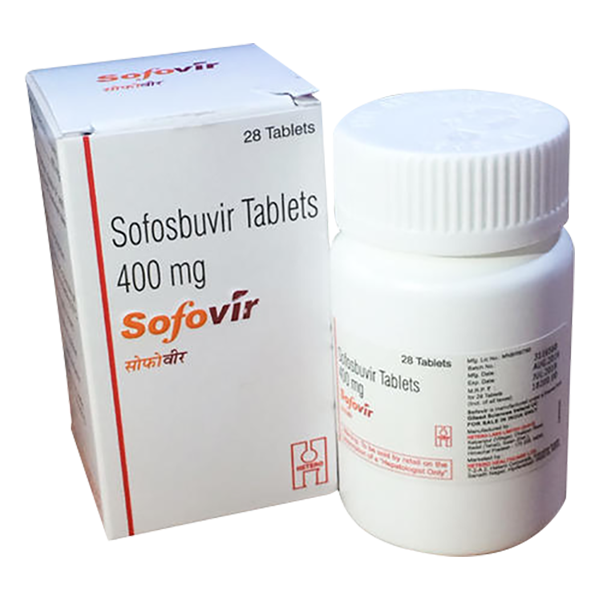
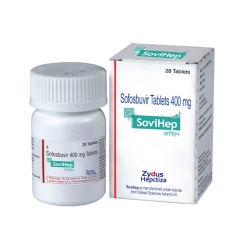
View larger Sofovir (Sofosbuvir 400mg)
New product
Indications & Dosage
Sofovir is a hepatitis C virus (HCV) nucleotide analog NS5B polymerase inhibitor indicated for the treatment of:
Adult patients with genotype 1, 2, 3 or 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis as a component of a combination antiviral treatment regimen.
Pediatric patients 12 years of age and older or weighing at least 35 kg with genotype 2 or 3 chronic HCV infection without cirrhosis or with compensated cirrhosis in combination with ribavirin.
Sofovir is a generic prescription medicine from Hetero, which is one of the biggest pharmaceutical company in India.
Sofovir (Sofosbuvir 400mg) Dosage:
Indicated for treatment of chronic hepatitis C virus (HCV) infection as a component of a combination antiviral regimen for patients with HCV mono-infection and HCV/HIV-1 coinfection
Treatment regimen and duration are dependent on both viral genotype and patient population
Genotype 1 or 4: 400 mg PO qDay plus ribavirin and peginterferon alfa for 12 weeks; may consider sofosbuvir plus ribavirin for 24 weeks in genotype 1 patients ineligible to receive peg-interferon-based regimen
Genotype 2: 400 mg PO qDay plus ribavirin for 12 weeks
Genotype 3: 400 mg PO qDay plus ribavirin for 24 weeks
Patients with hepatocellular carcinoma awaiting liver transplantation
- For prevention of post-transplant HCV reinfection
- 400 mg PO qDay plus ribavirin for up to 48 weeks or until the time of liver transplantation, whichever occurs first
Ribavirin dosage regimen with sofosbuvir (genotypes 1, 2, 3, and 4)
- Take with food
- <75 kg : 500 mg PO BID
- ≥75 kg: 600 mg PO BID
- Renal impairment (CrCl ≤50 mL/min): Reduce dose (see prescribing information)
Peginterferon alfa regimen with sofosbuvir (genotype 1 or 4)
- Peginterferon alfa 2a: 180 mcg SC weekly
- Peginterferon alfa 2b: 1.5 mcg/kg/week SC; not to exceed 150 mcg/week
- Renal impairment (CrCl ≤50 mL/min): Reduce dose (see prescribing information)
Reduction of sofosbuvir dose not recommended
Discontinue sofosbuvir therapy if the agents used in combination are discontinued
Genotypes 1 and 4
- Serious adverse reactions potentially related to peginterferon alfa and/or ribavirin: Should reduce or discontinue dose of peginterferon alfa and/or ribavirin following the recommendations of their respective prescribing information
Genotypes 2 and 3
- Serious adverse reaction potentially related to ribavirin: Modify or discontinue ribavirin dose
- Hemoglobin <10 g/dL without cardiac disease: Reduce ribavirin dose to 600 mg/day PO divided BID with food
- Hemoglobin <8.5 g/dL without cardiac disease: Discontinue ribavirin
- Cardiac disease and hemoglobin decreased ≥2 g/dL during 4 week period: Reduce ribavirin dose to 600 mg/day PO divided BID with food
- Cardiac disease and hemoglobin <12 g/dL despite 4 weeks at reduced dose: Discontinue ribavirin
- After discontinuing the dose may attempt to restart ribavirin at 600 mg PO divided bid and further increase it to 800 mg PO divided bid; increasing the dose to 1000-1200 mg/day not recommended
Renal impairment
- Mild or moderate: No adjustments required
- Severe (eGFR <30 mL/min/1.73 m²) or ESRD: No dosage recommendation can be given owing to higher exposures (up to 20-fold) of the predominant sofosbuvir metabolite
Hepatic impairment
- Mild, moderate, or severe (Child-Pugh Classes A, B or C): No dose adjustments required
- Decompensated cirrhosis: Not established
Efficacy has been established in combination with peginterferon alfa and ribavirin in HCV genotypes 1, 2, 3, and 4 infected subjects including hepatocellular carcinoma meeting Milan criteria (awaiting liver transplantation) and those with HCV/HIV-1 coinfection
Must not be used as monotherapy; if peginterferon alfa or ribavirin is discontinued for any reason, sofosbuvir must also be discontinued
Test all patients for evidence of current or prior hepatitis B virus (HBV) infection before initiating treatment with HCV direct acting antivirals (DDAs)
Sofovir (Sofosbuvir 400mg) side Effects:
can cause serious side effects, including: • Hepatitis B virus reactivation: Before starting treatment with SOVALDI, your healthcare provider will do blood tests to check for hepatitis B virus infection. If you have ever had hepatitis B virus infection, the hepatitis B virus could become active again during or after treatment of hepatitis C virus with Hepcvir (Sofosbuvir 400mg). Hepatitis B virus becoming active again (called reactivation) may cause serious liver problems including liver failure and death. Your healthcare provider will monitor you if you are at risk for hepatitis B virus reactivation during treatment and after you stop taking Hepcvir (Sofosbuvir 400mg).
Sofovir (Sofosbuvir 400mg) PI
For more Prescribing information,please ask for “FDA Prescribing Information” from our customer supporter.
Additional Info
| Package Quantity | 28 |
| Warnings | Don't take the drug without consulting a qualified doctor or physician |
| Suggested use | Follow the doctor's advice |
| By symptom | Sofosbuvir 400mg is a hepatitis C virus (HCV) nucleotide analog NS5B polymerase |
| Delivery & Returns | 7-14 Days |
Reviews
Accessories
Customers who bought this product also bought:
-

Hepcdac (Daclatasvir 60mg)
Hepcdac(Daclatasvir 60mg)
$79.00



















Very grateful
I've read a lot of forums and online pharmcy about the Sovaldi treatment but my experience after my 3rd week went south, difficult moving, loss of strength, vomiting, nausea, fatigue, no appetite, different muscle pains, back and side aches; at first I said this is a cake walk compare to the interferon. But I'm still thankful & had a clear test, one more month to go. I must say Indian generic drugs are really helpful. I'm very grateful.